ELIANA
Efficacy
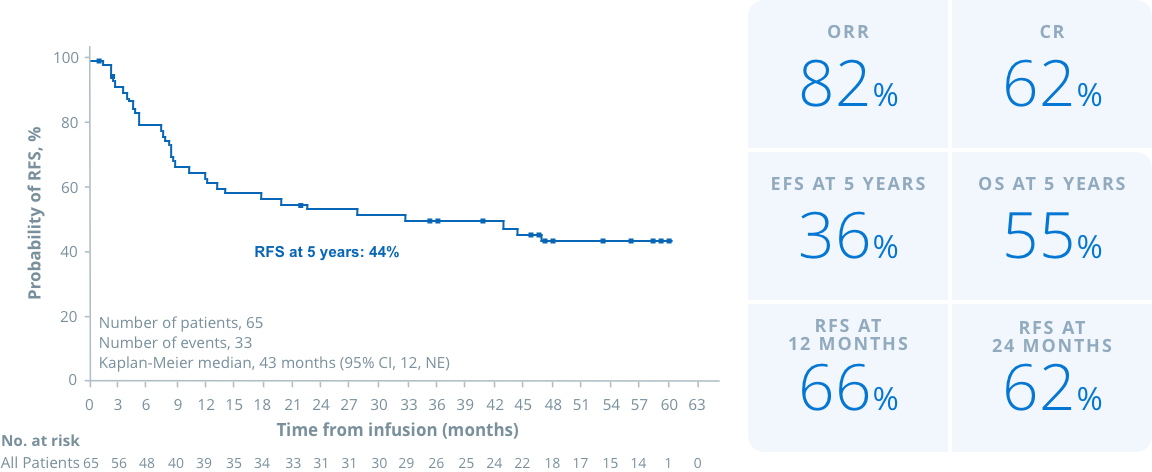
RFS
| Safety (within first 8 weeks after infusion) | |
|---|---|
| CRSGrade ≥3 | % |
| NEUROLOGICAL EVENTSGrade 3* | % |
Tisa-cel has shown sustained clinical benefit over 5 years in heavily pretreated p/AYA patients with ALL in ELIANA
*No grade 4 neurological events.
Efficacy
EFS
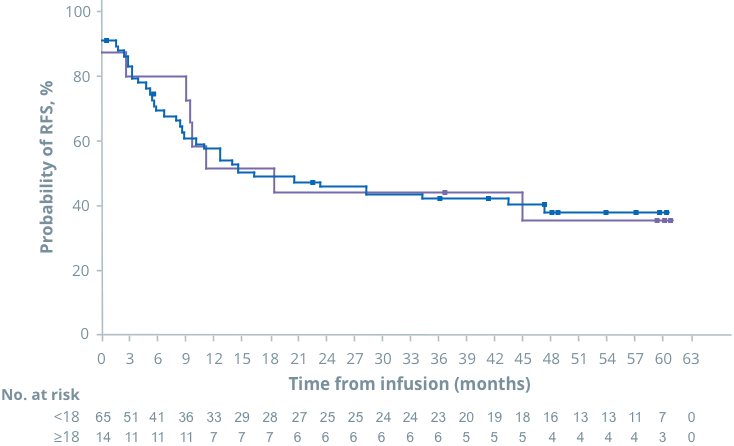
OS
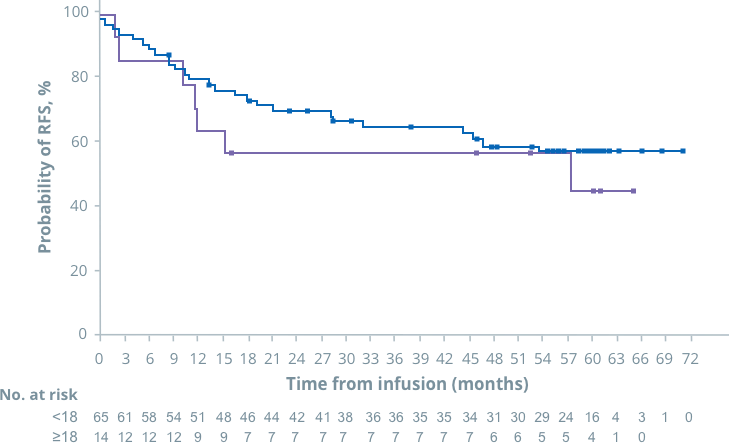

In ELIANA, similar outcomes were seen in patients <18 and ≥18 years1
Data collection
April 2015–present
25 study sites in 11 countries across North America, Europe, Asia, and Australia2
Median follow-up
months
No. of patients infused with tisa-cel
| Median age (range) years | 11 (3−24) |
| Gender (male/female) | 57/43% |
| Bone marrow status>M1 (>5% blasts)Blast count | 100%74% |
| CNS statusCNS-1CNS-2CNS-3 | 85%13%1% |
| Prior therapies (median)Prior blinatumomabPrior inotuzumab | 3 (1−8)NRNR |
| Prior HSCT | 61% |
| Primary refractoryFirst relapse≥2 relapses | 8%NRNR |
Patient
summary
Your patient summary will be shown here as you save you patient selections
This is where your patient summary will be shown. If you would like more tailored data, create a patient profile
Start a patient profileALL PATIENT
Patient profile
- Incomplete
Treatment history
- Incomplete